
Long term experiments for continuous conversion of HCl to Cl2 by using Mn2O3 as intermediate in a fixed bed reactor indicate that over 90% of HCl could be converted to Cl2 on stream of 30 h. PCBs are, because of their nature (high K ow values, hydrophobic and lipophilic), frequently attached to particles and removed to the bottom of. The X-ray diffraction (XRD) crystallite characterization results indicate the high conversion in each step under the optimum experimental conditions. The larger the number of chlorine atoms in the molecule, the less soluble is the compound in water and the less volatile and more persistent (or less prone to degradation) it is in the environment. (3) The MnO compound is oxidized by air to yield Mn2O3. It is commonly used as a bleach. It is usually handled as an aqueous solution.
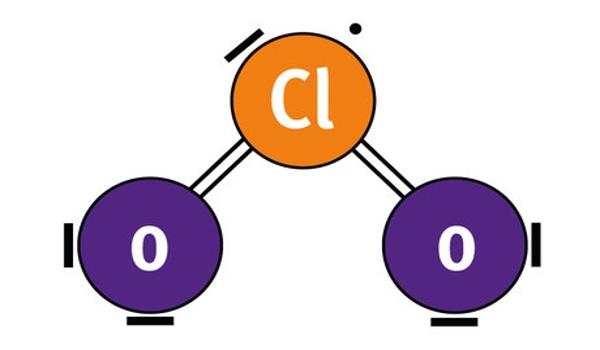
(2) Produced MnCl2 is oxidized by water steam to produce MnO at 450 ☌. Chlorine dioxide is a chemical compound with the formula ClO 2 that exists as yellowish-green gas above 11 ☌, a reddish-brown liquid between 11 ☌ and 59 ☌, and as bright orange crystals below 59 ☌. Determine the class of the compound, which contains carbon, hydrogen, chlorine, and oxygen, and exhibits the infrared spectrum below. (1) HCl steam is decomposed by intermediate Mn2O3 to produce Cl2 and MnCl2 at 500 ☌. The production of Cl2 from HCl with Mn compound as an intermediate and atmospheric steam is a feasible and recyclable process.ĪB - A new process is developed by using compound Mn as intermediate to produce Cl2 from HCl, with the following steps. The X-ray diffraction (XRD) crystallite characterization results indicate the high conversion in each step under the optimum experimental conditions. (2) Produced MnCl2 is oxidized by water steam to produce MnO at 450 ☌. After some general observations respecting the action of chlorine upon compounds containing carbon, and more especially upon car-buretted hydrogen gas. N2 - A new process is developed by using compound Mn as intermediate to produce Cl2 from HCl, with the following steps. All rights reserved.Ĭopyright 2015 Elsevier B.V., All rights reserved.
© 2014 The Chemical Industry and Engineering Society of China, and Chemical Industry Press. T1 - Clean production of chlorine from hydrogen chloride with Mn-compound as intermediate


 0 kommentar(er)
0 kommentar(er)
